Pharmacological control of therapeutic transgene expression
Major neurodegenerative disorders like Alzheimer´s or Parkinson´s disease are characterized by a multifaceted etiology and disease progression. Thus, it is unlikely that a single target will be identified, modification of which might halt disease progression or even revert symptoms in the majority of idiopathic patients. To circumvent this issue, neurotrophic factors (NTFs), that are able to stimulate multiple survival-promoting pathways are considered a valuable option to prevent further loss of neurons and even to restore neuronal functionality. However, the enormous potency of these molecules is a solid basis for side effects as well. As NTFs cannot cross the blood-brain-barrier, their expression within the CNS by means of gene therapy is currently considered the optimal route of delivery. As a drawback, gene therapy in its current layout is an irreversible process, meaning that in case of excess supply of NTFs, expression cannot be stopped, nor can the expression level be regulated according to individual patient´s needs.
In order to provide an alternative, we have developed and further optimized an AAV vector system that takes advantage of pharmacological control over transgene expression. The FDA-approved human drug Mifepriston (Mfp), a synthetic steroid, controls expression of the neurotrophic factor GDNF through the so-called “GeneSwitch”, enabling us to tightly control the level and duration of GDNF expression in the brain. The system has proven to work favorably in a rat model of Parkinson´s disease, providing robust recovery from motor impairments (Maddalena et al, Mol Ther Nucl Acids, 2013; Tereshchenko et al, Neurobiol Dis, 2014; Chen et al, Exp Neurol, 2018). While in the immune-privileged environment of the human brain it is unlikely that the short yeast-derived peptide component of the “GeneSwitch” provokes immunological consequences, we still consider it to be necessary to conduct immunological studies in non-human primates, which are underway to demonstrate safety. Prove of absence of peripheral immunity against the “GeneSwitch” would also enable use of this regulated gene therapy concept in organs outside the CNS.
Pharmacological control of neurotrophic factor expression
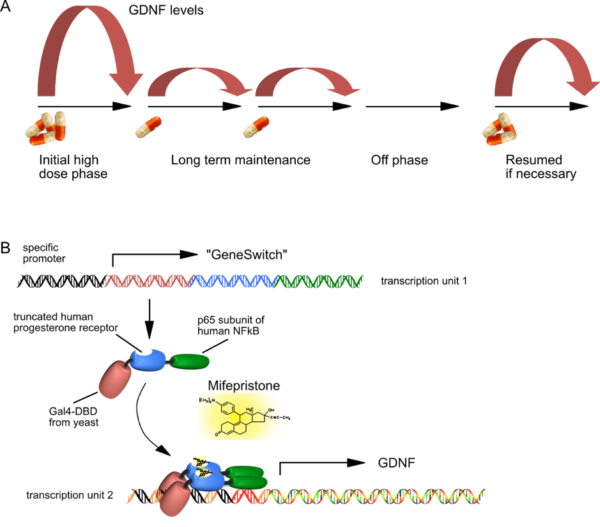
- A) The principle of regulated GDNF expression is shown to demonstrate that different levels of Mifepristone can induce GDNF levels over two orders of magnitude.
- B) Basic principle of “GeneSwitch”-regulated expression. Both transcription units fit into a single AAV vector genome, and need a certain spatial configuration in order to allow for high-level but background-free GDNF expression. The “GeneSwitch” fusion protein consists primarily of human components, except for about 70 amino acids of the yeast DNA binding domain. Therefore, immunological studies in immunocompetent organs of non-human primates must now prove that this peptide will not be immunogenic in humans.